A strong signal for quality and safety
June 2025 – bredent medical stands for purity, the highest product quality and consistent scientific validation. The award of the CleanImplant seal for the whiteSKY and copaSKY implant systems as well as HELBO photodynamic therapy confirms these high production standards and the reliable purity of bredent implants.
The independent CleanImplant Foundation uses high-resolution scanning electron microscopy (SEM) and extensive elemental analysis to test dental implants and related systems and is internationally recognised as the benchmark for the purity and quality of implantable systems. As a result, the requirements of the “Trusted Quality” seal go far beyond regulatory standards. The fact that all three bredent medical systems meet these requirements underlines the high security and quality of the company’s portfolio.


whiteSKY: proven biocompatibility
After 2022, the whiteSKY metal-free zirconium oxide implant was once again awarded the CleanImplant seal for the period 2025-2027. This re-certification demonstrates the consistently high quality of the system, which has been proven since 2006. whiteSKY combines high stability due to its one-piece design with the excellent biocompatibility of zirconium oxide – ensuring rapid osseointegration and long-term stable results. In particular, the Alveo and Tissue Line variants have been developed to meet both aesthetic and functional requirements in immediate and late care.
copaSKY: Bone Growth Concept for optimal osseointegration
The copaSKY implant system, on the other hand, was awarded the CleanImplant quality seal for the first time and thus meets all requirements for clinical cleanliness and outstanding material purity. The combination of microstructured backtaper, subcrestal positioning of the implants and narrow, tailored abutments as a further development of the platform switch – combined as the Bone Growth Concept – forms the basis of the system. This concept creates optimal conditions for bone adhesion and peri-implant mucosal sealing.
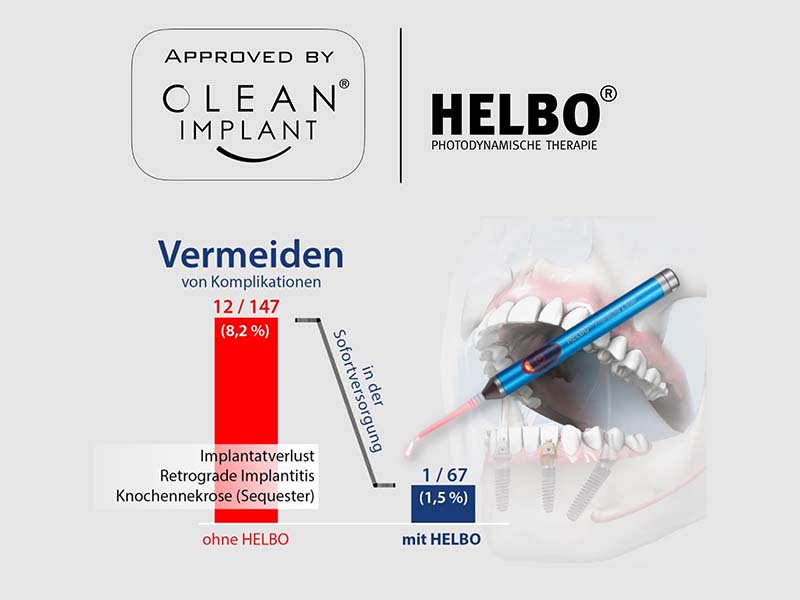
HELBO: Gentle therapy based on science
After reviewing extensive clinical data, HELBO photodynamic therapy (aPDT) was awarded the “Approved by CleanImplant” label for the first time – as the first non-implantological system from bredent medical. The award recognises the antimicrobial effect, biological compatibility and the consistent scientific validation of the HELBO method. aPDT targets pathogenic germs and supports the treatment of periodontitis and periimplantitis – as a gentle and substance-saving addition to conventional procedures. As part of implant treatment, it helps to create optimal conditions for immediate implantation or augmentation by disinfecting the extraction alveoli. It can also be used effectively for mucositis and thus helps to protect implants from periimplantitis in the long term.
With the CleanImplant awards, bredent medical is once again positioning itself as a technology-driven quality leader in the implantological field. The award of the CleanImplant certificates to whiteSKY, copaSKY and HELBO is more than a quality seal – it is a clear statement for uncompromising hygiene, the highest manufacturing quality and scientifically based product development. This underlines bredent medical’s aspiration to design modern implantology with certainty, systematics and substance.
stay tuned
Subscribe to our bredent group Press Mailing List
